Global Rivaroxaban Market is estimated to be valued at USD 17.9 Mn in 2025 and is expected to reach USD 28.3 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Rivaroxaban is gaining popularity as an anticoagulant for treating various medical conditions like deep vein thrombosis, pulmonary embolism and reducing the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation. The market is witnessing steady growth owing to rising geriatric population who are prone to cardiovascular diseases and growing awareness among physicians regarding advantages of rivaroxaban over warfarin. Additionally, patent expiry of major drugs in the next few years and the introduction of generic versions of rivaroxaban will provide opportunities for greater market penetration in low and middle-income countries.
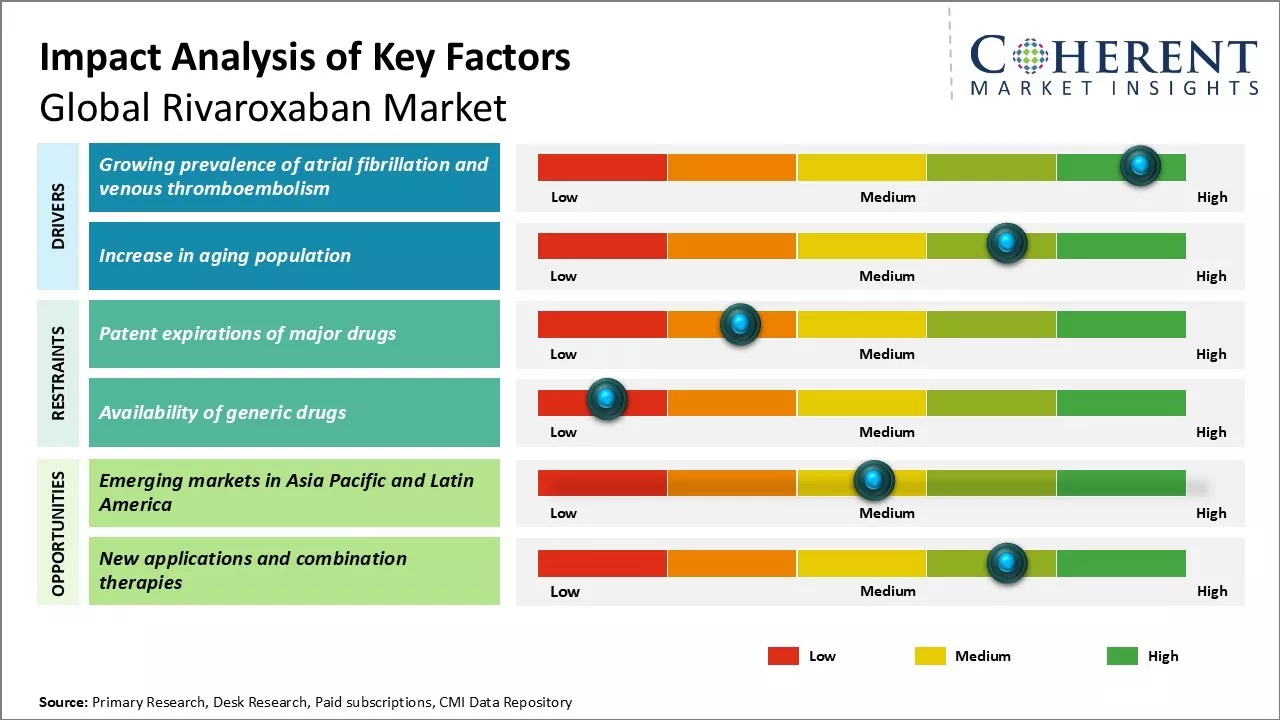
Growing prevalence of atrial fibrillation and venous thromboembolism
The growing prevalence of atrial fibrillation and venous thromboembolism is projected to significantly drive the demand for rivaroxaban in the coming years. Atrial fibrillation and venous thromboembolism have been on a steady rise globally over the past few decades. According to various studies and research papers, the incidence rate of atrial fibrillation is rising rapidly mainly due to aging population globally and rising prevalence of other cardiovascular disorders. Atrial fibrillation is associated with five times higher risk of stroke if left untreated. Similarly, venous thromboembolism including both deep vein thrombosis and pulmonary embolism has been rising steadily over the years. Prolonged immobilization from surgery, injuries or medical conditions increases risks for venous thromboembolism. With sedentary lifestyles and urbanization, rates of obesity have jumped manifold which is another contributing factor for venous thromboembolism.
Rivaroxaban has emerged as one of the preferred options for treatment and prevention of recurrent events of atrial fibrillation and venous thromboembolism due to its efficacy and safety profile. It provides an effective alternative to warfarin with predictable pharmacokinetics eliminating the need for regular blood monitoring. Rivaroxaban has been proven to be as effective as warfarin in reducing risks of stroke in atrial fibrillation with significantly lower risks of bleeds. Similarly, for the treatment of deep vein thrombosis and pulmonary embolism, rivaroxaban has shown benefits over standard therapy of subcutaneous low molecular weight heparins followed by VKAs. The increasing awareness about benefits of rivaroxaban and limitations of warfarin have paved the path for higher uptake of this novel oral anticoagulant. With rising disease prevalence, it can be expected that rivaroxaban demand will multiply in the coming years.
Get actionable strategies to beat competition: Download Free Sample
Increase in aging population
Aging population is another notable driver which is projected to fuel higher demand for rivaroxaban in global markets. Medical studies indicate that risks of both atrial fibrillation and venous thromboembolism increases significantly with age. The population aged 60 years and above are more predisposed to these conditions. According to data, people aged 80 years and above have five times higher likelihood of atrial fibrillation compared to general population. Similarly, incidence of venous thromboembolism is highest among people aged 60-79 years. With improvements in public health, hygiene and medical advances, average life expectancy has risen steeply globally. A larger section of the population is now surviving beyond 60 years of age. According to estimates by the United Nations, over next three decades number of elderly aged 60 years and above will nearly double to reach around 2 billion worldwide. This demographic transition has direct implications on disease epidemiology and pharma markets. The large elderly population base will encompass higher patient pool for atrial fibrillation and venous thromboembolism in turn driving prescriptions for oral anticoagulants including rivaroxaban. Apart from disease prevalence, aging population also prefers novel oral anticoagulants over vitamin K antagonists due to better safety, efficacy and convenience. The proliferation of geriatric population worldwide hence acts as a prominent growth lever for rivaroxaban market in long term.
Key Takeaways from Analyst:
The global rivaroxaban market is expected to witness steady growth over the forecast period driven by the rising prevalence of chronic diseases like diabetes and coronary artery disease which require anticoagulant therapy. Rivaroxaban has emerged as a major oral anticoagulant alternative to warfarin for treating conditions like pulmonary embolism and stroke prevention. Its once-daily dosing offers better patient compliance compared to warfarin. North America currently dominates the market owing to the increasing uptake of novel oral anticoagulants over warfarin. However, Asia Pacific is likely to be the fastest growing regional market due to rise in geriatric population in countries like China and India which are more prone to thromboembolic disorders.
The market also faces few challenges. Biosimilar competition from new pipeline drugs may dampen sales. Stringent regulations for drug approval poses restraint. Additionally, high costs and lack of awareness in developing nations also hamper market growth to some extent. However, ongoing clinical trials evaluating rivaroxaban’s efficacy in new therapeutic areas like acute coronary syndrome provide opportunities to expand indications. Furthermore, combination therapy approaches with antiplatelets could widen patient pool if proven safe.
Market Challenges: Patent expirations of major drugs
One of the major challenges faced by the global rivaroxaban market is the patent expirations of major drugs in the next few years. Rivaroxaban is currently marketed under the brand names Xarelto and Klorix by Bayer and Janssen respectively. However, the patents for these drug formulations will expire between 2023 and 2026. This will pave the way for cheaper generic variants of rivaroxaban to enter the market. The introduction of low-cost generics is likely to significantly reduce the prices of rivaroxaban products. This may negatively impact the revenues of major manufacturers who have developed and marketed the branded formulations of the drug. The companies need to devise new strategies to sustain their sales volumes and market share in a highly competitive post-patent expiry environment.
Market Opportunities: Emerging Markets in Asia Pacific and Latin America
The global rivaroxaban market has potential opportunities in the emerging economies of Asia Pacific and Latin America. In these regions, the prevalence of cardiovascular and thromboembolic disorders is continuously rising due to rapid urbanization, growing geriatric population, changing lifestyles, and increasing risk factors like obesity and smoking. This has led to a surge in demand for novel oral anticoagulants like rivaroxaban. Additionally, governments in Asia Pacific and Latin American countries are undertaking various healthcare reforms and spending more on public healthcare facilities. This is encouraging patients to opt for prescribed medications rather than alternative therapies. Market players can leverage these favorable macroeconomic factors by investing in these high potential emerging markets through strategies like regional partnerships and acquisitions. This will allow them to access new customer segments and drive future growth.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
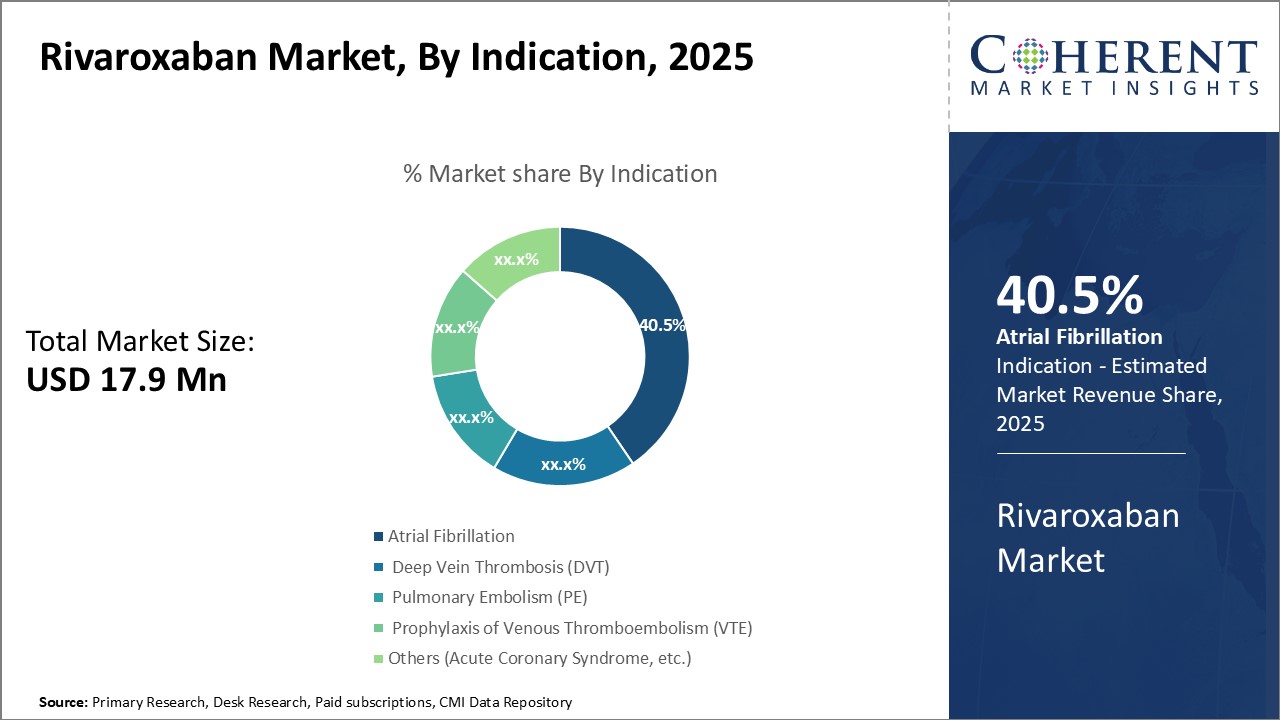
By Indication - Growing Prevalence of Atrial Fibrillation Drives the Segment
In terms of Indication, the Atrial Fibrillation segment is estimated to hold the highest share of the market with 40.0 % in 2025 owing to the growing prevalence of atrial fibrillation globally. Atrial fibrillation affects over 30 million people worldwide and is a major public health concern. It is associated with higher risks of stroke, heart failure and other cardiac issues. As the aging population continues to expand in developing nations, the prevalence of AFib is projected to increase significantly. Older age is a key risk factor for developing AFib.
Moreover, improved diagnosis and awareness have also led to more cases being detected early. Advancements in diagnostic technologies like mobile cardiac outpatient telemetry devices are enabling better screening of asymptomatic AFib patients in communities. This has raised overall diagnosis rates.
Rivaroxaban is widely used as one of the first line therapies for stroke prevention in non-valvular AFib due to its comparable efficacy and safety to warfarin with fewer drug and food interactions. Its fixed dosage regimen also offers better medication adherence. These benefits have driven the growing adoption of rivaroxaban among AFib patients.
By Formulation - Convenience and Compliance Drive Tablets Segment
In terms of Formulation, the Tablets segment is estimated to hold the highest share of the market with 59.5% in 2025. Compared to suspensions, tablets offer superior convenience of oral administration without needing to be mixed with water. This fixed dosage form is preferred by most patients and healthcare providers.
Tablets also aid better compliance to the prescribed medication regimen compared to suspensions which may precipitate or separate in storage. The fixed content uniformity of tablets allows consistent dosing of rivaroxaban everyday which is important for maintaining therapeutic drug levels and preventing thromboembolic events.
Additionally, the ease of portability and storage stability of tablets over suspensions make them a preferred choice for patients to maintain medication adherence during travel or outdoors. This supports the higher uptake of rivaroxaban in tablet formulation.
By Patient Type - Needs of Adult Patients Drive the Adult Segment
In terms of Patient Type, the Adults segment is estimated to hold the highest share of the market with 62.5% in 2025. This is primarily owed to the specific medical needs of adult patients treated with rivaroxaban. Conditions like AFib, DVT and PE which rivaroxaban is prescribed for are predominantly observed in adult and geriatric populations.
Age-related physiological changes also influence the pharmacokinetic and pharmacodynamic response to rivaroxaban in adults. Hence, extensive clinical research has been conducted to establish dosage regimens tailored for appropriate efficacy and safety in adult patients.
Since pediatric patients have restricted medical conditions indicated for rivaroxaban and lack established dosage guidelines, its utilization remains mainly focused on adults. Also, caregiver involvement is necessary for medication adherence in children, which adults can independently oversee. All these factors propel the higher penetration of rivaroxaban among adult patients.
Need a Different Region or Segment? Download Free Sample
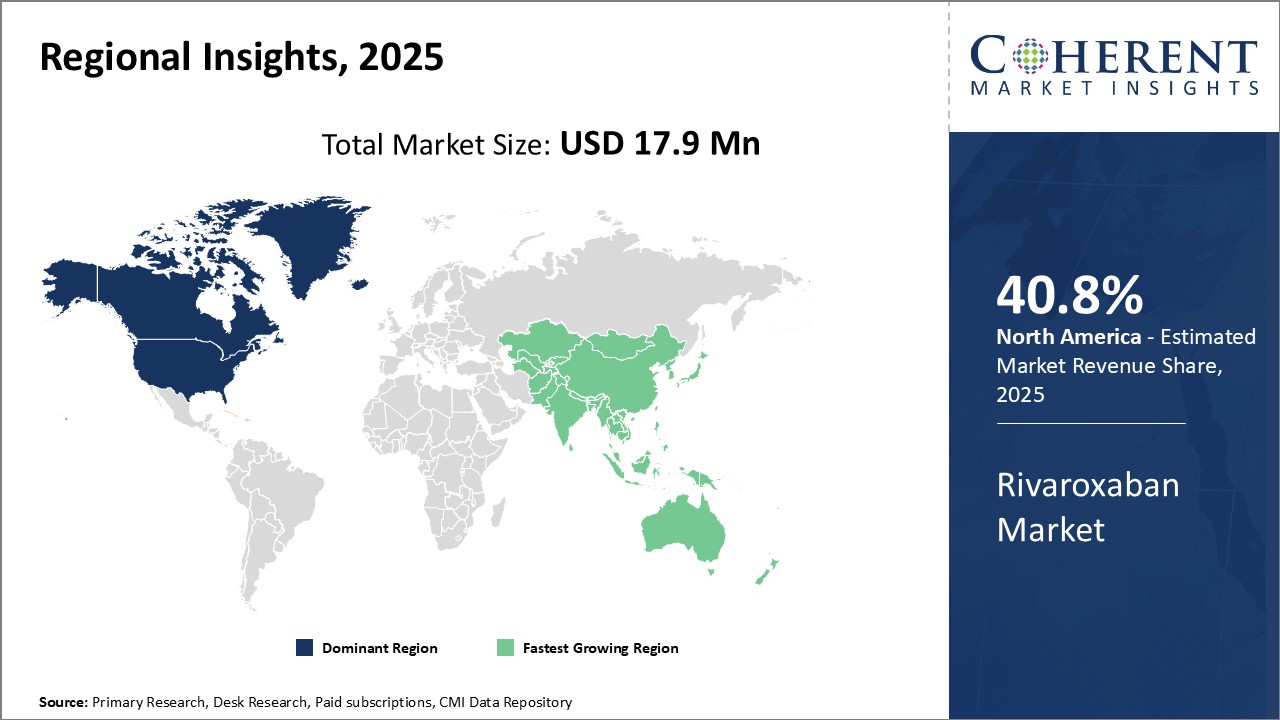
The North American region currently dominates the global rivaroxaban market with an estimated share of 40.8% in 2025. The presence of major pharmaceutical players in the U.S. and Canada is a key factor driving the large market size of the region. Companies like Bayer, Janssen, and Pfizer have established strong manufacturing and distribution networks in North America for Rivaroxaban and its variants which has ensured strong product availability. In addition, North America is known to be an early adopter of new drugs and therapies. The favorable regulatory environment allows for faster market approvals, which pharmaceutical companies leverage to gain an early foothold in the region. There is also greater awareness and acceptance of new oral anticoagulants like Rivaroxaban among physicians and patients in North America compared to other regions of the world.
The Asia Pacific region is projected to experience the fastest growth in the global rivaroxaban market over the coming years. Countries like India, China, Japan, and South Korea are expected to be the major contributors to the rising demand. Rapid economic development and rising healthcare expenditure has increased the affordability of new medicines. At the same time, a growing aging population and rising incidence of cardiovascular diseases and blood clots have also augmented the potential patient pool requiring anticoagulation treatment in Asia Pacific. The presence of key generic drug manufacturers is also improving access to cheaper Rivaroxaban alternatives. With lower manufacturing and labor costs, these companies cater to the large price-sensitive local markets as well as export to other regions. Strengthening healthcare infrastructure, collaborations between international and local players, and rising medical tourism are some of the factors expected to further propel the APAC Rivaroxaban market during the forecast period.
Rivaroxaban Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 17.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 28.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
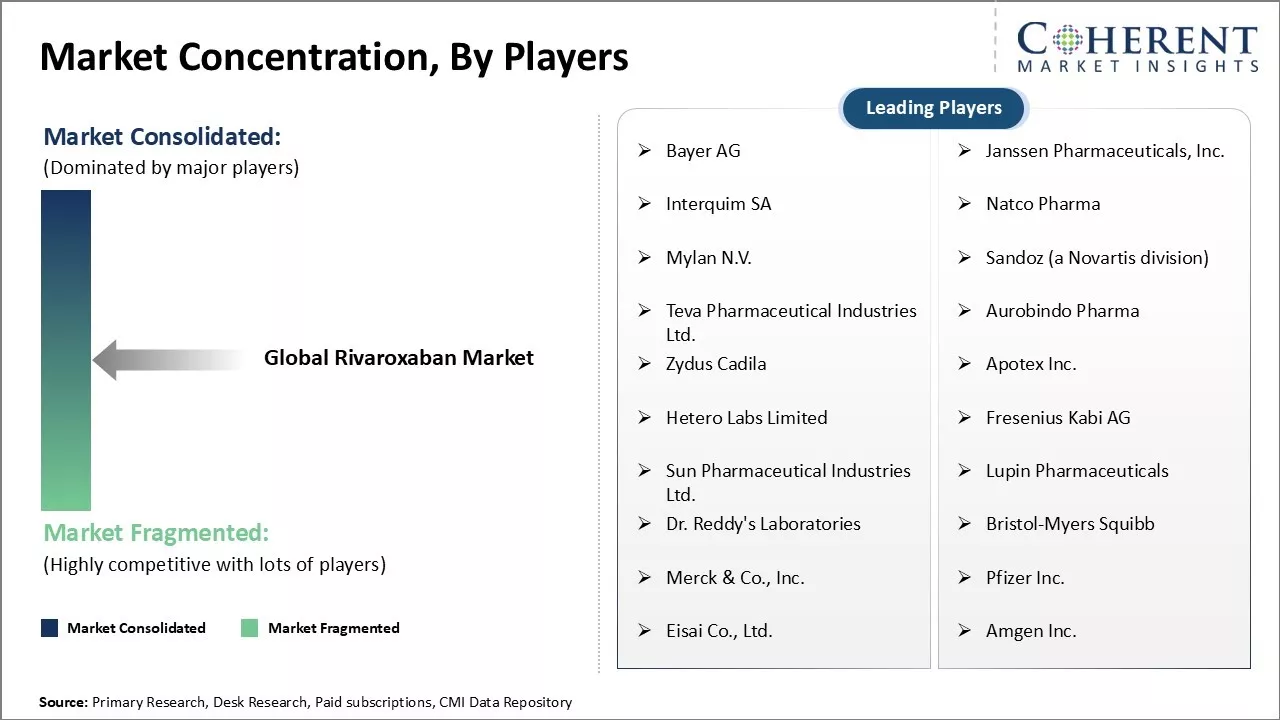
Bayer AG, Janssen Pharmaceuticals, Inc., Interquim SA, Natco Pharma, Mylan N.V., Sandoz (a Novartis division), Teva Pharmaceutical Industries Ltd., Aurobindo Pharma, Zydus Cadila, Apotex Inc., Hetero Labs Limited, Fresenius Kabi AG, Sun Pharmaceutical Industries Ltd., Lupin Pharmaceuticals, Dr. Reddy's Laboratories, Bristol-Myers Squibb, Merck & Co., Inc., Pfizer Inc., Eisai Co., Ltd., and Amgen Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients